Polonium
84
Po
Golongan
16
Periode
6
Blok
p
Proton
Elektron
Neutron
84
84
126
Sifat Umum
Nomor atom
84
Massa atom
[210]
Nomor massa
210
Kategori
Metaloid
Warna
Perak
Radioaktif
Ya
Dinamai dari Polandia, negara asal Madam Curie
Struktur kristal
Kubik Sederhana
Sejarah
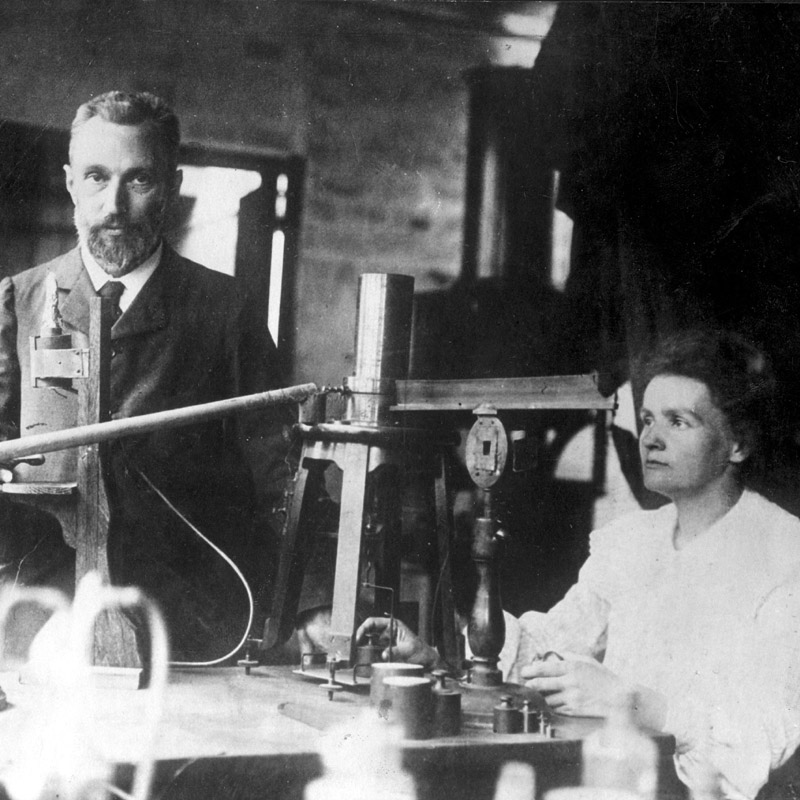
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
Elektron per kulit
2, 8, 18, 32, 18, 6
Konfigurasi elektron
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
Sifat Fisika
Fase
Solid
Kepadatan
9,196 g/cm3
Titik lebur
527,15 K | 254 °C | 489,2 °F
Titik didih
1235,15 K | 962 °C | 1763,6 °F
Kalor peleburan
13 kJ/mol
Kalor penguapan
100 kJ/mol
Kapasitas kalor molar
- J/g·K
Kelimpahan di kerak bumi
n/a
Kelimpahan di alam semesta
n/a
Nomor CAS
7440-08-6
Nomor PubChem CID
n/a
Sifat Atom
Jari-jari atom
168 pm
Jari-jari kovalen
140 pm
Elektronegativitas
2,00 (Skala Pauling)
Potensi Ionisasi
8,417 eV
Volume atom
22,23 cm3/mol
Kondusivitas termal
0,2 W/cm·K
Bilangan oksidasi
-2, 2, 4, 6
Aplikasi
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium is highly dangerous and radioactive
Isotop
Isotop stabil
-Isotop tidak stabil
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po