Radon
86
Rn
Golongan
18
Periode
6
Blok
p
Proton
Elektron
Neutron
86
86
136
Sifat Umum
Nomor atom
86
Massa atom
[222]
Nomor massa
222
Kategori
Gas mulia
Warna
Tak berwarna
Radioaktif
Ya
Namanya berasal dari radium; awalnya disebut niton, dari kata Latin nitens yang berarti berkilau
Struktur kristal
n/a
Sejarah
Radon was discovered in 1900 by Friedrich Ernst Dorn in Halle, Germany.
He reported some experiments in which he noticed that radium compounds emanate a radioactive gas.
In 1910, Sir William Ramsay and Robert Whytlaw-Gray isolated radon, determined its density, and determined that it was the heaviest known gas.
He reported some experiments in which he noticed that radium compounds emanate a radioactive gas.
In 1910, Sir William Ramsay and Robert Whytlaw-Gray isolated radon, determined its density, and determined that it was the heaviest known gas.
Elektron per kulit
2, 8, 18, 32, 18, 8
Konfigurasi elektron
[Xe] 4f14 5d10 6s2 6p6
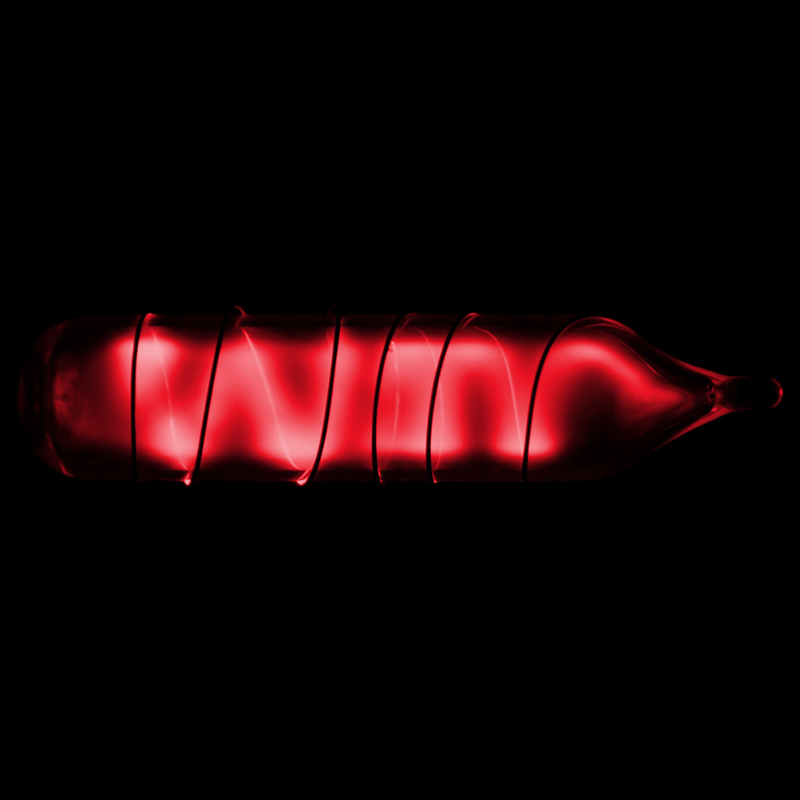
Upon condensation, radon glows because of the intense radiation it produces
Sifat Fisika
Fase
Gas
Kepadatan
0,00973 g/cm3
Titik lebur
202 K | -71,15 °C | -96,07 °F
Titik didih
211,3 K | -61,85 °C | -79,33 °F
Kalor peleburan
3 kJ/mol
Kalor penguapan
17 kJ/mol
Kapasitas kalor molar
0,094 J/g·K
Kelimpahan di kerak bumi
n/a
Kelimpahan di alam semesta
n/a
Nomor CAS
10043-92-2
Nomor PubChem CID
24857
Sifat Atom
Jari-jari atom
120 pm
Jari-jari kovalen
150 pm
Elektronegativitas
-
Potensi Ionisasi
10,7485 eV
Volume atom
50,5 cm3/mol
Kondusivitas termal
0,0000364 W/cm·K
Bilangan oksidasi
2, 4, 6
Aplikasi
Radon is used in hydrologic research that studies the interaction between ground water and streams.
Radon has been produced commercially for use in radiation therapy.
Radon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers.
Radon has been produced commercially for use in radiation therapy.
Radon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers.
Radon is highly radioactive and a carcinogen
Isotop
Isotop stabil
-Isotop tidak stabil
195Rn, 196Rn, 197Rn, 198Rn, 199Rn, 200Rn, 201Rn, 202Rn, 203Rn, 204Rn, 205Rn, 206Rn, 207Rn, 208Rn, 209Rn, 210Rn, 211Rn, 212Rn, 213Rn, 214Rn, 215Rn, 216Rn, 217Rn, 218Rn, 219Rn, 220Rn, 221Rn, 222Rn, 223Rn, 224Rn, 225Rn, 226Rn, 227Rn, 228Rn