Radium
88
Ra
Golongan
2
Periode
7
Blok
s
Proton
Elektron
Neutron
88
88
138
Sifat Umum
Nomor atom
88
Massa atom
[226]
Nomor massa
226
Kategori
Logam alkali tanah
Warna
Perak
Radioaktif
Ya
Dari kata Latin radius yang berarti sinar
Struktur kristal
Kubik Berpusat-badan
Sejarah
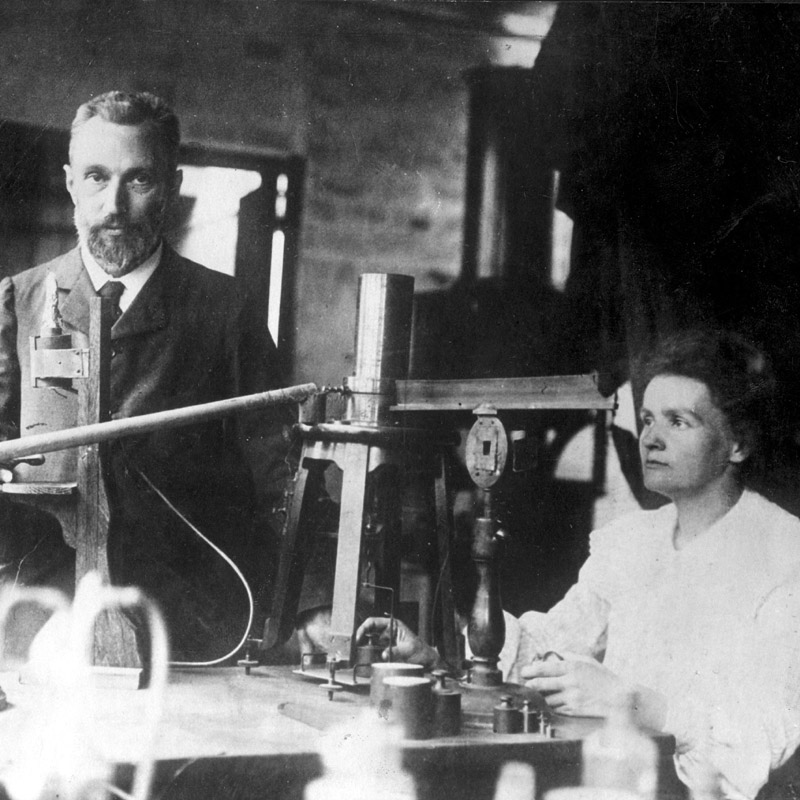
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Elektron per kulit
2, 8, 18, 32, 18, 8, 2
Konfigurasi elektron
[Rn] 7s2
Radium imparts a carmine red color to a flame
Sifat Fisika
Fase
Solid
Kepadatan
5,5 g/cm3
Titik lebur
973,15 K | 700 °C | 1292 °F
Titik didih
2010,15 K | 1737 °C | 3158,6 °F
Kalor peleburan
8 kJ/mol
Kalor penguapan
125 kJ/mol
Kapasitas kalor molar
- J/g·K
Kelimpahan di kerak bumi
9,9×10-12%
Kelimpahan di alam semesta
n/a
Nomor CAS
7440-14-4
Nomor PubChem CID
6328144
Sifat Atom
Jari-jari atom
-
Jari-jari kovalen
221 pm
Elektronegativitas
0,9 (Skala Pauling)
Potensi Ionisasi
5,2784 eV
Volume atom
45,20 cm3/mol
Kondusivitas termal
0,186 W/cm·K
Bilangan oksidasi
2
Aplikasi
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Isotop
Isotop stabil
-Isotop tidak stabil
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra