Aktinium
89
Ac
Golongan
n/a
Periode
7
Blok
f
Proton
Elektron
Neutron
89
89
138
Sifat Umum
Nomor atom
89
Massa atom
[227]
Nomor massa
227
Kategori
Aktinida
Warna
Perak
Radioaktif
Ya
Dari kata Yunani aktis, aktinos, yang berarti berkas atau sinar
Struktur kristal
Kubik Berpusat-muka
Sejarah
André-Louis Debierne, a French chemist, discovered actinium in 1899.
He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium.
Friedrich Oskar Giesel independently discovered actinium in 1902 as a substance being similar to lanthanum.
He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium.
Friedrich Oskar Giesel independently discovered actinium in 1902 as a substance being similar to lanthanum.
Elektron per kulit
2, 8, 18, 32, 18, 9, 2
Konfigurasi elektron
[Rn] 6d1 7s2
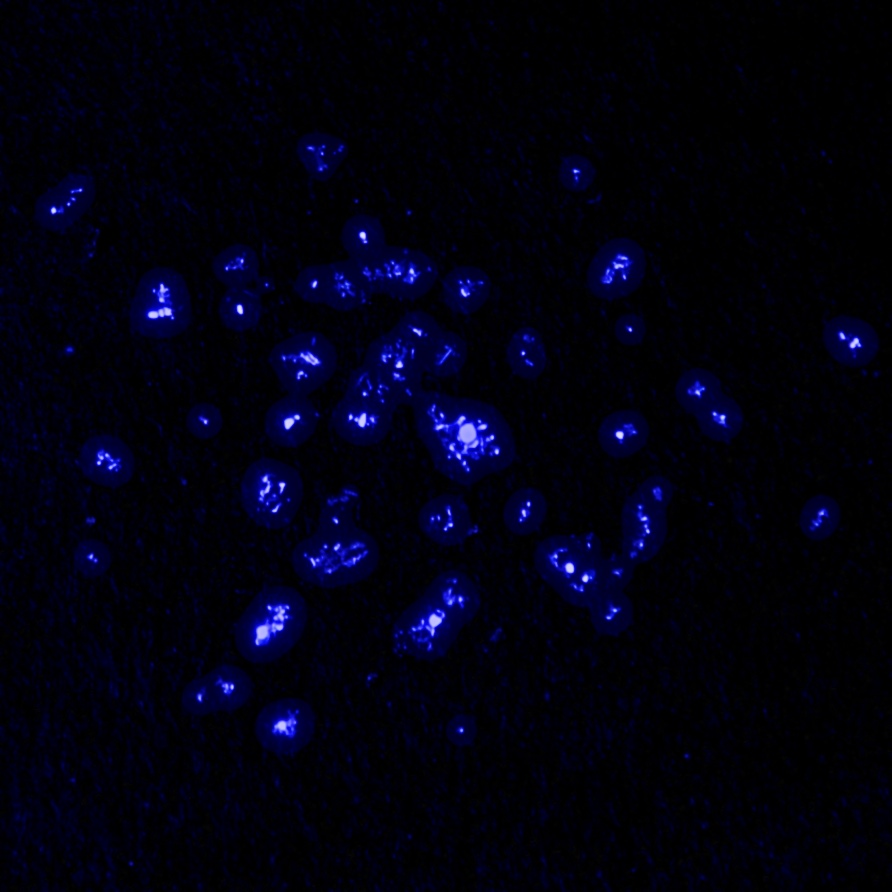
Actinium glows in the dark with a pale blue light
Sifat Fisika
Fase
Solid
Kepadatan
10,07 g/cm3
Titik lebur
1323,15 K | 1050 °C | 1922 °F
Titik didih
3471,15 K | 3198 °C | 5788,4 °F
Kalor peleburan
14 kJ/mol
Kalor penguapan
400 kJ/mol
Kapasitas kalor molar
0,12 J/g·K
Kelimpahan di kerak bumi
n/a
Kelimpahan di alam semesta
n/a
Nomor CAS
7440-34-8
Nomor PubChem CID
n/a
Sifat Atom
Jari-jari atom
-
Jari-jari kovalen
215 pm
Elektronegativitas
1,1 (Skala Pauling)
Potensi Ionisasi
5,17 eV
Volume atom
22,54 cm3/mol
Kondusivitas termal
0,12 W/cm·K
Bilangan oksidasi
3
Aplikasi
Actinium is used as an active element of radioisotope thermoelectric generators, for example in spacecraft.
The medium half-life of 227Ac makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters.
225Ac is applied in medicine to produce 213Bi in a reusable generator or can be used alone as an agent for radiation therapy.
The medium half-life of 227Ac makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters.
225Ac is applied in medicine to produce 213Bi in a reusable generator or can be used alone as an agent for radiation therapy.
Actinium is highly radioactive
Isotop
Isotop stabil
-Isotop tidak stabil
206Ac, 207Ac, 208Ac, 209Ac, 210Ac, 211Ac, 212Ac, 213Ac, 214Ac, 215Ac, 216Ac, 217Ac, 218Ac, 219Ac, 220Ac, 221Ac, 222Ac, 223Ac, 224Ac, 225Ac, 226Ac, 227Ac, 228Ac, 229Ac, 230Ac, 231Ac, 232Ac, 233Ac, 234Ac, 235Ac, 236Ac