Protaktinium
91
Pa
Golongan
n/a
Periode
7
Blok
f
Proton
Elektron
Neutron
91
91
140
Sifat Umum
Nomor atom
91
Massa atom
231,03588
Nomor massa
231
Kategori
Aktinida
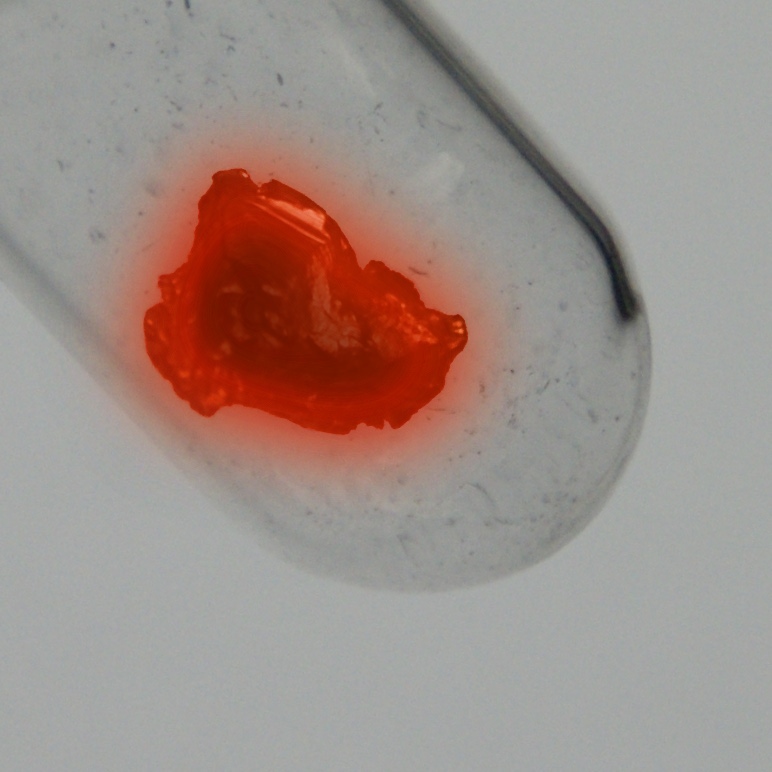
Warna
Perak
Radioaktif
Ya
Dari kata Yunani protos yang berarti pertama
Struktur kristal
Tetragonal Terpusat
Sejarah
In 1900, William Crookes isolated protactinium as an intensely radioactive material from uranium
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Elektron per kulit
2, 8, 18, 32, 20, 9, 2
Konfigurasi elektron
[Rn] 5f2 6d1 7s2
Protactinium is one of the rarest and most expensive naturally occurring elements
Sifat Fisika
Fase
Solid
Kepadatan
15,37 g/cm3
Titik lebur
1841,15 K | 1568 °C | 2854,4 °F
Titik didih
4300,15 K | 4027 °C | 7280,6 °F
Kalor peleburan
15 kJ/mol
Kalor penguapan
470 kJ/mol
Kapasitas kalor molar
- J/g·K
Kelimpahan di kerak bumi
9,9×10-13%
Kelimpahan di alam semesta
n/a
Nomor CAS
7440-13-3
Nomor PubChem CID
n/a
Sifat Atom
Jari-jari atom
163 pm
Jari-jari kovalen
200 pm
Elektronegativitas
1,5 (Skala Pauling)
Potensi Ionisasi
5,89 eV
Volume atom
15,0 cm3/mol
Kondusivitas termal
0,47 W/cm·K
Bilangan oksidasi
3, 4, 5
Aplikasi
Owing to its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
Protactinium is toxic and highly radioactive
Isotop
Isotop stabil
-Isotop tidak stabil
212Pa, 213Pa, 214Pa, 215Pa, 216Pa, 217Pa, 218Pa, 219Pa, 220Pa, 221Pa, 222Pa, 223Pa, 224Pa, 225Pa, 226Pa, 227Pa, 228Pa, 229Pa, 230Pa, 231Pa, 232Pa, 233Pa, 234Pa, 235Pa, 236Pa, 237Pa, 238Pa, 239Pa, 240Pa